Substance P/ Neurokinin-1 Receptor
I have been working on Neurokinin 1 Receptor, a G Protein Coupled Receptor in different pathologies since last 10 years. I have explored it in Sudden perinatal death, dental pain, COVID-19 and cancers. SP is abundantly contained in the fibers that innervate the dental pulp and dentin. SP has a high affinity for the NK-1 receptor, to which it binds preferentially. SP/NK-1R complex plays a key role in the interaction in the onset of pain and inflammation. The amount of SP released by each sensory fiber is further increased during inflammatory processes, which sustains the vicious circle that underlies inflammation.4 SP exerts its biological actions by binding to a high-affinity G-protein-coupled receptor denoted NK-1R, located on most inflammatory cells, such as mast cells and macrophages, and on other connective-tissue cells.
Aprepitant as a combinant with Dexamethasone reduces the inflammation via Neurokinin 1 Receptor Antagonism in Covid-19 patients: A novel therapeutic approach
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent for the coronavirus disease 2019 (COVID-19), is responsible for the present pandemic and global health crisis since December 2019. The WHO reports an estimated 64 million cases and 1.5 million deaths associated with COVID-19 ...[1]. Pakistan reports a total of 406,000 confirmed COVID-19 cases, with a case-fatality of 2.0% [2]. The absence of presently available COVID-19 vaccines demands novel treatment options in severe cases to reduce mortality. Convalescent plasma (CP) therapy is a form of passive immunization which involves the plasma infusion of patients suffering from active infection with plasma from recovered/recovering patients. CP therapy has shown satisfactory efficacy and safety in the past two decades for treating infectious disease, more recently against coronaviruses as with SARS and MERS [3], [4].CP therapy promises short-lived immunity that is rapidly acting, however, it risks of the transfusion of infectious agents, transfusion-associated circulatory overload (TACO) and transfusion-associated acute lung injury (TRALI) which is of particular concern in SARS, SARS-CoV-2 and MERS-CoV patients. Antibody-dependent enhancement (ADE) is another risk potentially caused by antibody therapies in SARS, MERS, RSV, measles and is of particular concern in COVID-19. To mitigate the risk of ADE, it is suggested that COVID-19 patients undergoing CP therapy should be infused with high titers of potent neutralizing antibodies rather than low doses of non-neutralizing antibodies [7]. The therapeutic potential and success of CP therapy in improving clinical outcomes of patients suffering with SARS-CoV and MERS-CoV has been demonstrated in case reports [4], [8]. A plausible immunomodulatory therapy building upon the outcome and success of CP therapy studies is the infusion of severe COVID-19 patients with intravenous immunoglobins (IvIg) to suppress viremia. Thus, concentrating convalescent plasma into anti-coronavirus hyper immune intravenous globulin (hIVIg) circumvents potential risks associated with conventional plasma therapy while allowing greater antibody activity per unit of volume. Furthermore, CP therapy has the added risk of facilitating pro-coagulatory activities in COVID-19 patients, who are already at high-risk of developing embolisms [10], while hIVIg therapy avoids this risk altogether, as it is a pure formulation containing only antibodies. Unlike convalescent plasma, small scale concentrates of immunoglobulin prepared from convalescent plasma collections provide higher potency and greater consistency than individual units. Potential drawbacks of employing CP therapy over immunoglobulin therapy include: • Immunoglobulin therapy is non-specific to patient blood group unlike CP therapy, which requires blood group matching prior to administration. • Immunoglobulin therapy only includes a pure formulation of antibodies reducing the risk of cross-reactions. In contrast, only 18% of plasma constitutes immunoglobulins required for passive immunization while the remainder contains proteins that may trigger reactogenicity and serum sickness in recipients. • A significantly lower dosage volume of immunoglobulins (3-5ml/day) is required as opposed to CP therapy (200-300ml plasma/patient). • Immunoglobulins are far less prone to sterility issues than plasma, which contains a high portion of proteins that carry risk of contamination. • Concentrated immunoglobulins are far more potent as they demonstrate a targeted response. In case of plasma, proteins fractions pose a delayed response. • Plasma therapy is subjected to moderate to severe patients specially, while all affected individuals can take benefit of immunoglobulin therapy because dose of immunoglobulins can be controlled. An open label Phase III clinical trial is ongoing ClinicalTrials.gov Identifier: NCT04548557
Intravenous immunoglobulins for the treatment of Covid-19 patients: a clinical trial
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent for the coronavirus disease 2019 (COVID-19), is responsible for the present pandemic and global health crisis since December 2019. The WHO reports an estimated 64 million cases and 1.5 million deaths associated with COVID-19... [1]. Pakistan reports a total of 406,000 confirmed COVID-19 cases, with a case-fatality of 2.0% [2]. The absence of presently available COVID-19 vaccines demands novel treatment options in severe cases to reduce mortality. Convalescent plasma (CP) therapy is a form of passive immunization which involves the plasma infusion of patients suffering from active infection with plasma from recovered/recovering patients. CP therapy has shown satisfactory efficacy and safety in the past two decades for treating infectious disease, more recently against coronaviruses as with SARS and MERS [3], [4].CP therapy promises short-lived immunity that is rapidly acting, however, it risks of the transfusion of infectious agents, transfusion-associated circulatory overload (TACO) and transfusion-associated acute lung injury (TRALI) which is of particular concern in SARS, SARS-CoV-2 and MERS-CoV patients. Antibody-dependent enhancement (ADE) is another risk potentially caused by antibody therapies in SARS, MERS, RSV, measles and is of particular concern in COVID-19. To mitigate the risk of ADE, it is suggested that COVID-19 patients undergoing CP therapy should be infused with high titers of potent neutralizing antibodies rather than low doses of non-neutralizing antibodies [7]. The therapeutic potential and success of CP therapy in improving clinical outcomes of patients suffering with SARS-CoV and MERS-CoV has been demonstrated in case reports [4], [8]. A plausible immunomodulatory therapy building upon the outcome and success of CP therapy studies is the infusion of severe COVID-19 patients with intravenous immunoglobins (IvIg) to suppress viremia. Thus, concentrating convalescent plasma into anti-coronavirus hyper immune intravenous globulin (hIVIg) circumvents potential risks associated with conventional plasma therapy while allowing greater antibody activity per unit of volume. Furthermore, CP therapy has the added risk of facilitating pro-coagulatory activities in COVID-19 patients, who are already at high-risk of developing embolisms [10], while hIVIg therapy avoids this risk altogether, as it is a pure formulation containing only antibodies. Unlike convalescent plasma, small scale concentrates of immunoglobulin prepared from convalescent plasma collections provide higher potency and greater consistency than individual units. Potential drawbacks of employing CP therapy over immunoglobulin therapy include: • Immunoglobulin therapy is non-specific to patient blood group unlike CP therapy, which requires blood group matching prior to administration. • Immunoglobulin therapy only includes a pure formulation of antibodies reducing the risk of cross-reactions. In contrast, only 18% of plasma constitutes immunoglobulins required for passive immunization while the remainder contains proteins that may trigger reactogenicity and serum sickness in recipients. • A significantly lower dosage volume of immunoglobulins (3-5ml/day) is required as opposed to CP therapy (200-300ml plasma/patient). • Immunoglobulins are far less prone to sterility issues than plasma, which contains a high portion of proteins that carry risk of contamination. • Concentrated immunoglobulins are far more potent as they demonstrate a targeted response. In case of plasma, proteins fractions pose a delayed response. • Plasma therapy is subjected to moderate to severe patients specially, while all affected individuals can take benefit of immunoglobulin therapy because dose of immunoglobulins can be controlled. An open label Phase III clinical trial is ongoing ClinicalTrials.gov Identifier: NCT04548557
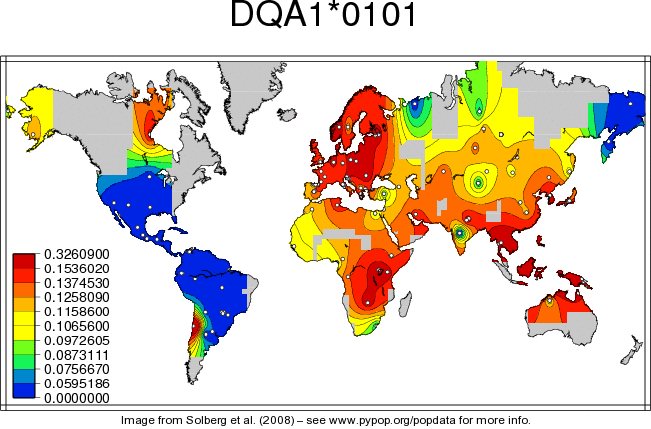
Associations between HLA allele frequencies and the development and/or severity of COVID-19
Since HLA plays a key role in the immune response of humans to pathogens. It has been hypothesized that the diversity seen in most polymorphic gene of HLA in different populations may have some association with the occurrence, susceptibility and presentation of COVID-19 [13].... The Human Leukocyte Antigen (HLA) system with its alleles has a pivotal genetic role in viral antigen presentation pathway determining the outcome of many viral diseases which confers difference in viral susceptibility and severity of disease. It has been studied that disease caused by the closely related SARS-CoV (25, 26) presented more severely among individuals with the genotype of HLA-B*46:01 genotype (26). Associations between viral diseases and HLA genotype is also seen in other unrelated viruses like hepatitis, severe acute respiratory syndrome (SARS) and human immunodeficiency virus 1 (HIV-1) as well [14, 15, 16]. For example patients infected with HIV, found to have association of HLA-A*0202 and HLA A*6802 with a significantly decreased rate of HIV-1 seroconversion [25]. While the clinical picture of COVID-19 pandemic continue to emerge, the immune response against SARS-CoV-2 predicts the role of individual genetic variability and leaves with substantial unanswered questions [26].

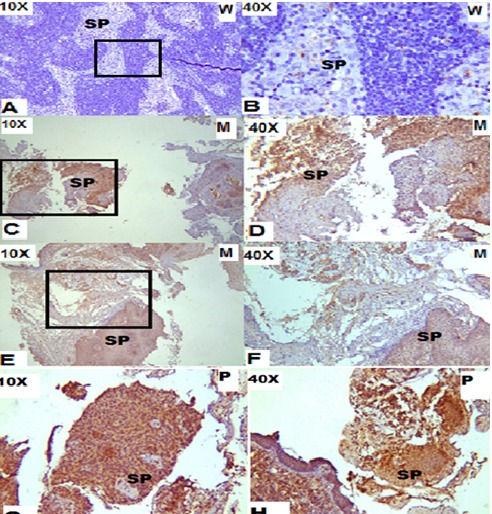
Association of Neurokinin-1 Receptor Expression with human dental pulp inflammation and Pain severity
Dental pain perception is due to an inflammatory reaction going on inside the tooth pulp.1 Neuropeptides are now considered major determinants of the inflammatory process in peripheral tissues. Neuropeptides take part in the process of transmission and modulation of pain and inflammatory process.... These neuropeptides include Substance P (SP), calcitonin gene-related peptide (CGRP), Neurokinin A (NK-A), Neuropeptide Y (NP-Y), and vasoactive intestinal polypeptide (VIP) among others. Substance P (SP)/Neurokinin-1 Receptor (NK-1R), induces inflammatory reactions in peripheral tissues including the dental pulp, but its regulatory effects in target tissues are dependent on receptor signaling. NK-1R expression in inflamed den¬tal pulps. SP/ NK-1R modulation may provide a novel approach for the treatment of pulpal inflammation and pain.

Immunohistochemical expression and association of Neurokinin-1 Receptor with progression of different cancers
NK-1R is expressed in associated with different carcinoma with a significantly increased expression during clinical advanced stage. It should be explored and antagonists may serve as a therapeutic regimen for the treatment of bladder carcinoma. It may also serve as a diagnostic marker to differentiate the stages as well as to diagnose the carcinoma....
Figure A) Well Differentiated OSCC, SP weak positive, +1 intensity, Intercellular bridges and moderate amount of cytoplasm at 10X, B) at 40X, C) Moderately differentiated OSCC, SP intermediate positive, +2 intensity, strands in epithelium and underline epithelium cells D) at 40X, E) Moderately differentiated OSCC, SP positive, +2 intensively of SP expression, F) at 40X, G) Poorly differentiated OSCC, SP strongly positive, intensity +3, showing 80-90% turn or in the cells and maximum atypia at 10X, H) Poorly differentiated OSCC, SP strongly positive, +3 intensity, sheet of neoplastic cells and atypia, Bundle of m malignant cells

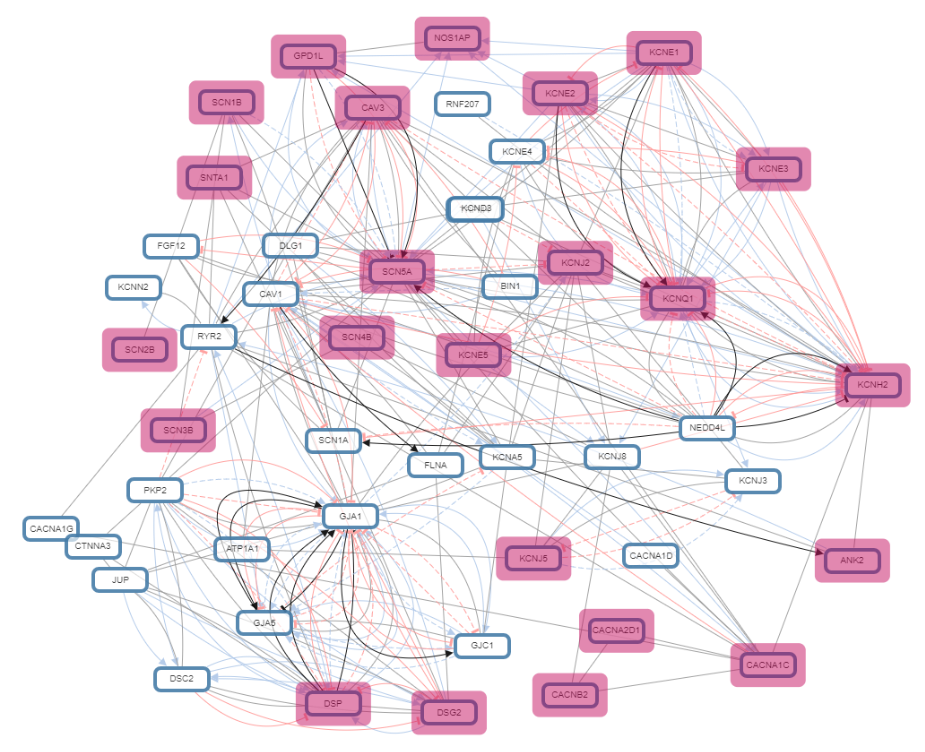
Comprehensive analysis of genes associated with Sudden Infant Death Syndrome: A computational approach
Sudden infant death syndrome is a tragic incident which remains a mystery even after post-mortem investigation and thorough researches. ...This 0. 1analysis is based on the genes reported in molecular autopsy studies conducted on SIDS so far. Molecular and computational approaches are a step forward towards exploration of these sad demises. It is so far a new arena but seems promising to dig out the root cause of sids in the years to come.
Ongoing Research Projects
- To determine the efficacy of aprepitant, a Neurokinin 1 Receptor Antagonist, in combination with Dexamethasone, as a therapeutic tool against cytokine storm and respiratory failure in COVID_19 patients
- Intravenous immunoglobulins for the treatment of COVID-19 patients: A structured summary of a study protocol for a randomized controlled trial
- Associations between HLA allele frequencies and the development and/or severity of COVID-19
- Repurposing Neurokinin-1 Receptor, Aprepitant in combination with corticosteroid, Dexamethosone as a therapeutic regimen for severe to critically ill COVID_19 patients.
- Substance P: an emerging trouper for progression of endometrial carcinoma
- Immunolocalization of NRF2 transcription factor in Brain Tumors
- Protein arginine methyltransferase 5 molecular and immunohistochemical expression in WHO grade IV Astrocytomas and Glioblastomas
- Immunohistrochemical expression of NDRG2 in Astrocytomas and Glioblastomas.
- Substance P and Neurokinin 1 Receptor expression in tissue, plasma and expression of Tachykinin 1 gene in urothelial carcinoma patients
- Immunohistochemistry and ELISA based Substance P / Neurokinin 1 Receptor Expression in Gastric, Endometrial, Breast, Renal Cell Carcinoma and Lung and Tachykinin 1 Gene mutations
- Transient receptor potential cation channel subfamily V member 1 immunolocalization in breast
- prostate, ovarian and oral squamous cell carcinoma
- Voltage-gated sodium channel Nav 1.9 immunolocalization in different cancers



